COVID-19
The pandemic is over. The COVID-19 death toll is down thanks to the vaccines we have to protect us, but we need to be vigilant! COVID-19 is still out there and changing. It may be that we will have COVID-19 vaccines annually like we do the flu shot. Time will tell.
In the meantime, the good news is that in the U.S., vaccines are available to everyone 6 months and older! If you haven’t already, get the latest vaccine booster now!
Quick Links
FAQs | Vaccines Approved in the U.S. | Vaccines in Process | Vaccine Approval Process | Treatments
Questions About
COVID-19 Vaccines?
Can I Still Get Vaccinated?
Yes, in the United States, vaccination is available to everyone 6 months and older. Check with your doctor, your health plan or use Vaccines.gov to find out where you can be vaccinated now.
Can Children Get Vaccinated for COVID-19?
Yes, the Pfizer and Moderna vaccines are approved to use for those 6 months to 18 years though they may have different dosages (CDC). The other available vaccines are for those 18 and over. Watch for updates in available vaccines for children.
Should You Get a COVID-19 Booster Shot?
Everyone 6 years and older should get 1 update (bivalent) Pfizer-BioNtech or Moderna COVID-19 vaccine, regardless of whether you’ve received any vaccine.
For children 6-5 years who got Pfizer-BioNTech COVID-19
- Aged 6 Months through 4 years and you get 3 COVID-19 vaccine doses, including at least 1 updated COVID-19 dose.
- Aged 5 years and you get at least 1 updated COVID-19 vaccine dose.
For children 6-5 years who got Pfizer-BioNTech COVID-19
You need 2 Moderna COVID-19 vaccine doses, including at least 1 updated COVID-19 vaccine dose.
If you got the Novovax or Johnson & Johnson vaccines, they also have doses specific for your age, but do not have the updated bivalent doses available (CDC).
Should You Get the Updated COVID-19 Vaccine?
Where Do You Get the COVID-19 Vaccine?
The CDC refers people to the Vaccine Finder. All locations may not be listed. Be sure to ask your health plan or provider what might be closest. Pharmacies near you may also have vaccinations available (CDC). You can look up your local health office by scrolling to the bottom of the page found here. Most vaccination sites require you to make an appointment.
What Happens When You Get Vaccinated?
A vaccination is a simple injection (shot) in the arm. (Note, a nasal spray is available to healthy people 2 to 49 years old for the flu vaccine.)
After you get a vaccination, you may have minor reactions such as a sore arm or low-grade fever. These symptoms should go away within a few days at most. Remember, vaccines are continually monitored for safety, and like any medication, vaccines can cause side effects which are usually minor. However, a decision not to immunize also involves risks and could put you and your loved ones and others who come into contact with you at risk of contracting a potentially deadly disease (CDC).
Will You Be Immune Once You Get the Vaccine?
You will not immediately be immune after being vaccinated. It takes at least two weeks after the second dose of the Moderna and Pfizer vaccines and two weeks after the single dose of the Johnson & Johnson vaccine before you receive the full protection of those doses. You could still get COVID-19 because the vaccines are not 100% effective. In addition, the CDC also recommends one booster shot for those 5 and older and two booster shots for those 50 plus.
Immunocompromised: Those who are immunocompromised should get a 3rd dose at least 28 days after the 2nd dose of the Pfizer or Moderna vaccines and at least one booster. Visit the CDC and discuss the boosters with your doctor.
To learn more about the vaccines available, visit the COVID-19 Vaccine page on this website or visit the CDC.
Is There a Treatment for COVID-19?
In December 2021, the FDA gave Emergency Use Authorization (EUA) to Pfizer for Paxlovid. It is a pill taken orally. Paxlovid is the first treatment for COVID-19 to receive any type of authorization in the U.S. It is authorized to treat mild-to-moderate COVID-19 in adults and pediatric patients (12 years of age and older weighing about 88 pounds) who are at high risk for progression to severe COVID-19, including hospitalization or death. Paxlovid is available by prescription only and should be used as soon as possible after diagnosis and within five days of symptom onset. Paxlovid is NOT a substitute for vaccination (FDA). The CDC recommends everyone be vaccinated fully.
Will the Vaccines Protect You From the New Strains of COVID-19?
The Vaccines Approve in the U.S.
The first two COVID-19 vaccines, Pfizer and Moderna, available to help us fight COVID-19 are mRNA Vaccines. The specific codes that make COVID-19’s “spike” protein (which is what enables the virus to infect people) were isolated and copied as mRNA fragments, which is what cells use as instructions for making proteins. Those fragments are packaged into special molecules, then injected into the patient. Within the patient, the mRNA fragments enter the body’s factories. The factory “reads” the mRNA instructions and makes copies of the spike protein. Spike protein copies are partial and cannot, by themselves, cause harm, but they will trigger the body to make antibodies against the spike portion of the virus. Those antibodies protect patients from a COVID-19 infection (Forbes).
The third vaccine, by Johnson & Johnson, is a viral vector vaccine. A viral vector vaccine uses a virus, but one that is modified so it can’t cause harm in people to deliver the instructions to factories in the body to make a harmless part of the COVID-19 spike. That triggers an immune response in our body which teaches it how to respond if exposed to the real COVID-19 virus (CDC). Johnson & Johnson’s vaccine is recommended for people who had a severe reaction or severe allergy to an mRNA vaccine or any ingredient in Pfizer-BioNTech or Moderna vaccines.
The fourth vaccine, by Novavax Inc., is a protein subunit vaccine. A protein subunit vaccine contains little pieces of proteins from the COVID-19 virus. Like the mRNA vaccines, this is the “spike” protein; however, the difference is this vaccine also contains an adjuvant, which teaches the immune system how to react to the “spike.” With this vaccination, if your body contracts the real COVID-19 proteins, the immune system recognizes it immediately and knows how to fight it off. Protein subunit vaccinations do not contain any live virus and have been used for decades (CDC).
Click Below to Learn More
Pfizer / BioNTech Vaccine
The Pfizer/BioNTech vaccine (Pfizer) was the first to be authorized in the U.S. Data from their Phase 3 study of nearly 44,000 people (42% having diverse background) has shown their vaccine candidate to be 95% effective in helping to prevent COVID-19. This was consistent across age, race and ethnicity demographics. In adults over 65 years of age, effectiveness was over 94%.
Things to Know
This vaccine requires two doses, delivered by injection, three to four weeks apart. It is approved for those 5 years old and over. If you have an existing condition or have had a reaction to a vaccine, please consult your doctor. The vaccine does not contain eggs, latex or preservatives (CDC).
Side Effects
According to the FDA, the most commonly reported side effects were pain at the injection site, tiredness, headache, muscle pain, chills, joint pain, and fever. More people experienced these effects after the second dose than after the first dose, and they lasted several days.
Moderna Vaccine
The Moderna COVID-19 Vaccine is approved by the FDA. It is marketed as Spikevax for the prevention of COVID-19 in individuals 18 years of age and older (FDA). Early results from the Moderna clinical trials showed their vaccine to be “94.1% effective with more than 30,000 participants in the U.S. in the trial.” Its success was consistent across age, race and ethnicity, and gender demographics. The Cove study (Phase 3 trial) included those over 65, with chronic health conditions, and “individuals who self-identify as people of color” (Moderna).
Things to Know
The Moderna vaccine requires two doses, one month (28 days) apart. It is recommended for people aged 18 years and older. The vaccine does not contain eggs, preservatives or latex. You should not get the vaccine if you have had a severe allergic reaction (anaphylaxis) or an immediate allergic reaction—even if it was not severe—to the first dose or any ingredient in an mRNA COVID-19 vaccine (such as polyethylene glycol) (CDC).
Side Effects
Moderna is a mRNA vaccine. According to a Moderna news release, “no serious adverse events were noted in the trial." Reactions to the vaccine include potential pain, redness, and swelling in the arm and tiredness, headache, muscle pain, chills, fever and nausea in the rest of the body (CDC).
Johnson & Johnson Vaccine
The Janssen/Johnson & Johnson vaccine is the third vaccine approved for Emergency Use Authorization (EUA) by the FDA. In clinical trials it was reported this vaccine prevented hospitalization and death (J&J).
"The J&J vaccine was tested in more people who identified as Hispanic/Latino and Black or African American. It was also tested in more people who were 60 years or older and in those who had conditions such as heart disease, obesity, and diabetes" (Duke Health).
Things to Know
This vaccine is a viral vector vaccine. It is easier to store than the mRNA vaccines and requires only one dose. The vaccine is approved for people 18 years old and older. If you have previously had a reaction to a vaccine or to any ingredient in the vaccine, you should not get the J&J vaccine (J&J/CDC).
Side Effects
The most common side effects include injection site pain, headache, fatigue, muscle ache and nausea. These usually happen 1-2 days after being vaccinated. The symptoms are reportedly mild to moderately severe (FDA). However, according to the CDC, "women younger than 50 years old especially should be aware of the rare risk of blood clots with low platelets after vaccination, and that other COVID-19 vaccines are available where this risk has not been seen. If you received a J&J/Janssen vaccine, here is what you need to know. Read the CDC/FDA statement" (CDC).
Novavax Inc. Vaccine
The Novavax COVID-19 vaccination is approved by the FDA for Emergency Use Authorization for individuals 18 years of age and older as a two-dose primary series (FDA). Results from Novavax’s Phase 3 clinical trial called PREVENT-19 found “the Novavax COVID-19 Vaccine, Adjuvanted demonstrated 90.4% efficacy” with “29,960 participants aged 18 years and older in the U.S. and Mexico” (Novavax). Novavax Inc. is currently in trials to study the efficacy of their vaccine on children aged six months to 17 years old.
Things to Know
The vaccine requires two doses in the primary series, 3-8 weeks apart, but has not been approved for a booster dose. People who are severely immunocompromised are recommended to get their two doses three weeks apart. The vaccine does not contain any eggs, preservatives, latex, metals, tissues, or antibiotics (CDC).
Side Effects
According to Novavax Inc. the most common side effects experienced in Phase 3 were pain and tenderness at the injection site, fatigue, and muscle pain. These reactions were seen after the first and second dosage (Novavax).
COVID-19 Vaccine Boosters
The FDA and CDC are now recommending COVID-19 boosters for those 6 months and older (CDC).
If you received the two-shot series of the Pfizer or Moderna COVID-19 vaccine at least five (5) months ago, you can get a Pfizer booster shot if you are 5 or over, or the Moderna booster if you are 18 or over.
If you got the Johnson & Johnson single shot COVID-19 vaccine at least two (2) months ago, you can get a booster shot if you are 18 years or older (CDC).
You can get any of the COVID-19 vaccines authorized in the United States as a booster (CDC).
Immunocompromised.
Those who are immunocompromised should get a 3rd dose at least 28 days after the 2nd dose of the Pfizer or Moderna vaccines (CDC).
Fall 2022 Booster
Updated boosters are called “bivalent” because they protect against both the original virus and the Omicron variants (BA.4 and BA.5). The CDC recommends the bivalent booster for everyone 6 months and older.
Where Do You Get Booster Shots?
Most places that gave the original shots (doses) can give you a booster.
→ Search Vaccines.gov, or call 1-800-232-0233, to find locations near you.
→ Check your local pharmacy’s website to see if vaccination walk-ins or appointments are available.
Please check in your state or local health department for more guidance on receiving boosters in your state and region.
COVID-19 Treatments
There are several treatments for COVID-19. They include anti-virals, Evusheld, a pretreatment, and monoclonal antibodies (CDC). You can also visit what’s new in COVID-19 treatment at the NIH.
Click Below to Learn More
Anti-Virals
Paxlovid
In December 2021, the FDA gave Emergency Use Authorization (EUA) to Pfizer for Paxlovid Paxlovid is the first oral antiviral pill for COVID-19 to receive any type of authorization in the U.S.
Paxlovid is available by prescription only and should be used as soon as possible after diagnosis and within five day of symptom onset. Paxlovid is NOT a substitute for Vaccination (FDA). The CDC recommends everyone be vaccinated fully.
Who is eligible?
It is authorized to treat mild-to-moderate COVID-19 in adults and pediatric patients (12 years of age and older weighing about 88 pounds) who are at high risk from progression to sever COVID-19, including hospitalization or death.
Remdesivir
Remdesivir is an intravenous antiviral medication approved by the Food and Drug Administration (FDA) for the treatment of COVID-19 in adults and pediatric patients 28 days or more old and weighing 3 or more kilograms. In high-risk, non-hospitalized patients with mild to moderate COVID-19, Remdesivir should be started within 7 days of symptom onset and administered for 3 days. It can also be used for hospitalized adults (NIH). Talk to your doctor to see if this medication is right for you.
Lagevrio
The U.S. Food and Drug Administration (FDA) has issued an Emergency Use Authorization (EUA) for Lagevrio to treat mild-to-moderate COVID-19 in adults (18 or over) at high risk for severe COVID-19 including hospitalization or death, and for whom other COVID-19 treatment options approved or authorized by the FDA are not accessible or appropriate. Do not take Lagevrio if you are pregnant or may become pregnant (FDA).
Evusheld
For Those Immunocompromised Before They Contract COVID-19
Evusheld is a long-acting antibody for pre-exposure prophylaxis (PrEP) for the prevention of COVID-19 in immunocompromised patients under Emergency Use Authorization (EUA).
It is a combination of two potent, monoclonal antibodies administered in two intramuscular injections. EVUSHELD is designed to provide pre-exposure protection against COVID-19 to those who are not expected to mount an adequate immune response to authorized COVID-19 vaccines.
In multiple independent laboratory studies, EVUSHELD had neutralizing power against Omicron subvariants, as well as the original strain of COVID-19.
Who is eligible?
Immunocompromised adult and pediatric individuals 12 years of age and older (weighing at least 88 lbs) who are not infected with SARS-CoV-2 are eligible to receive EVUSHELD for pre-exposure (PrEP). For those who recently received a COVID-19 vaccine dose, whether it is a first, second, or booster dose, FDA advises to wait two weeks before administration of Evusheld.
There are a range of conditions that compromise the immune system, from underlying disease to immune suppressing treatments that qualify a patient to receive Evusheld; in addition, the rare individual for whom a COVID-19 vaccine causes a severe reaction is also a candidate. You can review the NIH (NIH Guidelines Panel’s prioritization) eligibility here, and please contact your doctor for more information. For more information go here.
Monoclonal Antibodies
Read More About them Here
The mAb treatment, bebtelovimab, can block the virus that causes COVID-19 from entering cells in your body and limit the amount of the virus within your body. This means you may have milder symptoms and may decrease the likelihood of you needing to stay in the hospital.
A mAb treatment may help people who are at high risk of getting more serious symptoms and have a positive COVID-19 test with symptoms for 7 days or less (bebtelovimab) or have been in close contact with someone who has recently tested positive. Talk to your doctor about this treatment option. To find a location to get bebtelvimab treatments visit here.
The NIH Guidelines Panel’s prioritization recommends bebtelovimab as an alternative therapy option. To be used when neither of the preferred treatment options are available, feasible to use, or clinically appropriate (NIH).
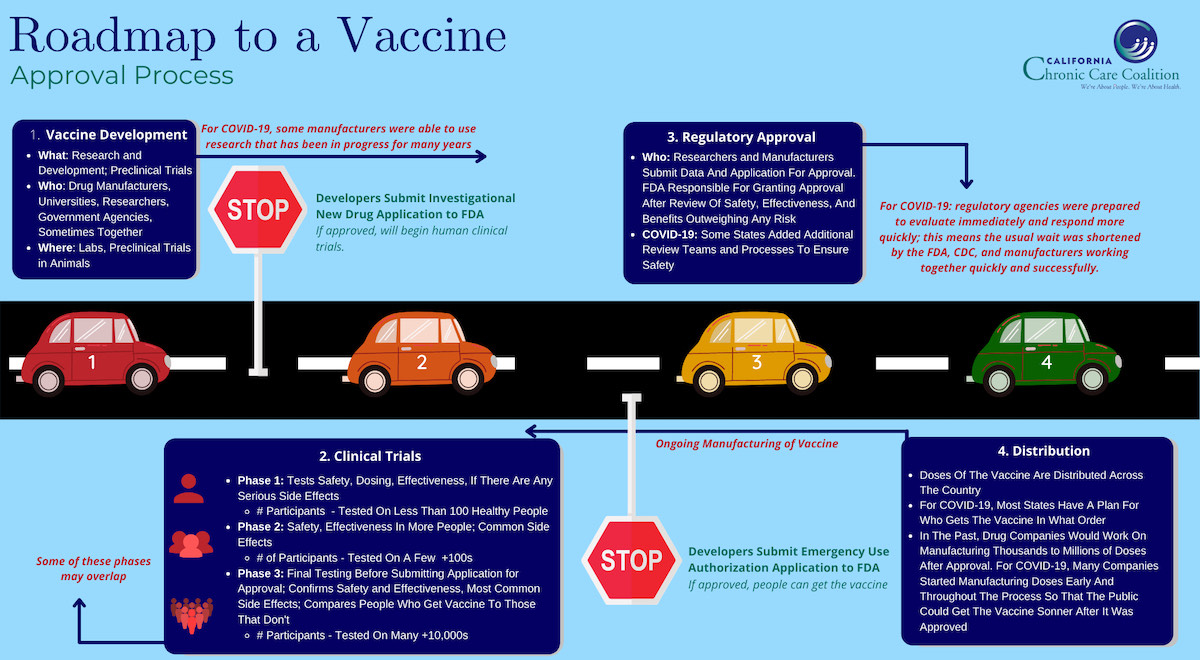
The COVID-19
Vaccine Approval Process
Operation Warp Speed identified the most promising potential vaccines and provided government support to lessen the time required to receive FDA approval and prepare for production and delivery of the vaccine. The vaccine manufacturers worked with the U.S. government on safety needs throughout the process. While the COVID-19 vaccine trial process has been thorough, it has been faster than normal largely because the three trial phases and manufacturing and delivery preparation have been done in an overlapping sequence. Also, mRNA vaccines — the first two vaccines created and submitted to the FDA by Pfizer and Moderna — are generally faster to create, test, and market.
The FDA has a Vaccine Advisory Committee that reviews all scientific data once a vaccine has been submitted and makes a recommendation to approve a vaccine or not. In the case of the COVID-19 vaccines, they were first recommended for Emergency Use Authorization.
Emergency Use Authorization (EUA) FDA Approval
An EUA makes a vaccine or medication available to the public faster than the traditional approval process, but full FDA approval still hinges on data that shows the vaccine is safe and effective. Since EUA is more limited than full approval, drug makers are expected to submit the vaccine for full approval later.
Once the FDA accepts a positive recommendation and the vaccine is approved for limited emergency use, the company can distribute it.
Through Operation Warp Speed, the federal government made investments in the manufacturing end of the vaccine development process, enabling faster distribution of the vaccine. The Department of Defense set up and put into action a plan to deliver the vaccine nationwide, with states determining where vaccines would be provided and in what priority.
Ver esta página en español